
The Annex 1 is a section of the European Union’s Good Manufacturing Practices (GMP) guidelines, dedicated to providing detailed guidance and information for manufacturers of sterile products.
The much-anticipated revised version of Annex 1 was published on August 25, 2022, and became effective a year later, on August 25, 2023.
This update reflects changes in manufacturing environments, seeking to facilitate implementation of innovative tools and advanced technologies. To ensure the highest standards of purity, quality, and patient safety, all manufacturers producing sterile products for the European market must adhere to the requirements outlined in Annex 1.
The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use.
Read the official European Union Guidelines here.
How can the UV222 Booth aid in complying with Annex 1 requirements?
The Annex 1 mandates that manufacturers of sterile products implement measures to minimize the risk of microbial, endotoxin/pyrogen, and particle contamination.
The UV222 Booth, utilizing Far-UVC technology, provides a rapid decontamination solution for cleanroom operators before they enter higher-grade cleanrooms.
Operators simply step into the UV222 Booth and activate it by waving at the touchless start module. This action triggers the inactivation of any microorganisms within seconds – whether bacteria, virus, or fungi – on their gowns, gloves, masks, or goggles. This process significantly lowers the risk of contamination excursions, preventing costly downtime and investigation, while aiding compliance with Annex 1’s stringent microbial contamination standards.
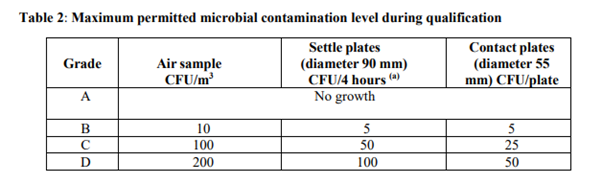
What are the maximum microbial contamination levels defined in Annex 1?
Annex 1 outlines maximum permitted microbial contamination levels for cleanrooms in section 4.31, Table 2, categorized by cleanroom grade. These microbial levels are measured in colony forming units (CFUs), which represent viable microorganisms capable of growth and colony formation. For the highest grade of cleanroom, Grade A, no microbial growth is allowed in any sample.
[https://health.ec.europa.eu/system/files/2022-08/20220825_gmp-an1_en_0.pdf Page 13]
Annex 1 part 4.31 The microbial contamination level of the cleanrooms should be determined as part of the cleanroom qualification. The number of sampling locations should be based on a documented risk assessment and the results obtained from room classification, air visualization studies and knowledge of the process and operations to be performed in the area. The maximum limits for microbial contamination during qualification for each grade are given in Table 2. Qualification should include both “at rest” and “in operation” states.
Learn more here
The UV222 Booth effectively inactivates microorganisms, preventing their growth. Thus, it supports adherence to Annex 1’s specified microbial contamination levels by ensuring that personnel entering high-grade cleanrooms do not contribute to the microbial burden.
Where does microbial contamination in cleanrooms originate from?
The primary source of contamination in cleanrooms is the operators themselves, despite strict protocols for gowning, behavior, and procedures [1]. Humans naturally shed viable microorganisms from their hair, skin, mouth, and nose. These microorganisms pose a contamination risk during aseptic donning process, as they can adhere to gowns, face masks, goggles, or gloves.
Integrating the UV222 Booth into this process allows for inactivation of contaminating microorganisms on gowning equipment before operators enter the higher-grade cleanroom areas.
What is Far-UVC technology?
Far-UVC technology’s antimicrobial efficacy is extensively documented in independent, peer-reviewed studies [2-4]. The UV222 Booth employs a specific wavelength of 222 nm, known to inactivate microorganisms while remaining safe for human exposure [5]. This wavelength is highly absorbed by proteins, preventing it from penetrating human skin or eyes.
The in light is generated by filtered excimer krypton-chloride (KrCl) lamps, with the filter ensuring that no wavelengths outside the 222 nm peak are emitted. The transmission of far-UVC light through cleanroom gowning material is less than 0.05%, resulting in an extremely low dose of only 0.0058 mJ/cm2 reaching the operator during a 30-second cycle in the UV222 Booth. This is more than 3750 times lower than the regulatory limits (threshold limit value) of 22 mJ/cm2.
In contrast to chemical disinfectants, the UV222 Booth does not produce harmful residues or environmental pollution, offering an eco-friendly and chemical-free decontamination alternative.
-
References
- Smith LML, C.; O' Driscoll, N. H.; Lamb A. J. Identification of Bacterial Isolates Recovered from the Surface of Cleanroom Operators’ Garments following Wear. European Journal of Parenteral and Pharmaceutical Sciences. 2022;27(3).
- Hessling M, Haag R, Sieber N, Vatter P. The impact of far-UVC radiation (200-230 nm) on pathogens, cells, skin, and eyes - a collection and analysis of a hundred years of data. GMS Hyg Infect Control. 2021;16:Doc07.
- Buonanno M, Ponnaiya B, Welch D, Stanislauskas M, Randers-Pehrson G, Smilenov L, et al. Germicidal Efficacy and Mammalian Skin Safety of 222-nm UV Light. Radiat Res. 2017;187(4):483-91.
- Narita K, Asano K, Naito K, Ohashi H, Sasaki M, Morimoto Y, et al. 222-nm UVC inactivates a wide spectrum of microbial pathogens. J Hosp Infect. 2020.
- Görlitz M, Justen L, Rochette PJ, Buonanno M, Welch D, Kleiman NJ, et al. Assessing the safety of new germicidal far-UVC technologies. Photochem Photobiol. 2023.
Never miss another article? Sign up for our newsletter.
 UV222™
UV222™ UV222 Linear
UV222 Linear UV222 Downlight
UV222 Downlight Vertex 222
Vertex 222.png) UV222 Pendant
UV222 Pendant.png) UV222 Booth
UV222 Booth.png) UV222 Step-On
UV222 Step-On.png) UV222 Cleanroom Downlight
UV222 Cleanroom Downlight UV222 Dual Downlight 60x60
UV222 Dual Downlight 60x60 UV222 Material Airlock
UV222 Material Airlock UV222 Ambulance
UV222 Ambulance UV222 Compact
UV222 Compact UV222 Industrial
UV222 Industrial




.jpg)
.jpg)

%20(3)%20(2).png)