UV222 Booth
Enter your cleanroom clean
The UV222 Booth from UV Medico is the only available solution that decontaminates gowned operators before entering the cleanroom


Designed to minimise environmental bioburden
Cleanrooms are spaces with a controlled level of contamination. They are designed to control and limit microbiological contamination when it represents a risk to product quality, patients, or consumers. Cleanrooms are necessary in many industries, from the automotive industry to pharmaceuticals, and are subject to a strict monitoring of pollutants in air and surfaces.
The UV222 Booth can help meeting the established limits for microbial contamination, by disinfecting gowned personnel right at the entrance of high-grade classified areas.
Using filtered Far-UVC, which is safe for use in occupied spaces, the UV222 Booth offers operator disinfection in under 30 seconds and an advanced touchless operation interface with built-in safety parameters. The UV222 Booth is a revolutionary addition to current contamination control measures in cleanrooms. It eliminates any residual microorganisms present on protective equipment*, ensuring a higher grade of cleanliness and a lower risk of product contamination.
The UV222 Booth can be delivered as a stand-alone solution, or as a customised version, encased in a stainless steel frame from floor to ceiling to fully integrate in any cleanroom.
-
Specifications
Specifications
Light source Krypton chloride excimer lamp Wavelength 222 nm Dose in 30 sec. (at centre) 12.0 mJ/cm²† Input voltage 230 V AC (50/60 Hz) Mode 30 sec. duty cycle† Max power consumption 3 kW Weight 430 kg (948 lbs)† Dimensions† L: 1,250 mm
W: min 1,453 mm
H: 2,170 mm(49.21 x 57.20 x 85.43 in)†
Operating temperature 0° to + 50°C (32° to 122°F)
Ambient humidity 5–90% RH † Values based on a custom booth with 156 Far UV-C light sources.
UV222 Booth can be customised upon request. -
Efficacy
Microorganism Type Time for decontamination (sec.)‡ Reduction in 30 sec. (%)‡ Escherichia coli Bacteria 4.78 >99,9 % Listeria monocytogenes Bacteria 8.15 >99,9 % Salmonella Typhimurium Bacteria 4.49 >99,9 % Staphylococcus aureus Bacteria 7.38 >99,9 % Candida albicans Fungi 22.37 95.4% Bacillus subtilis spores Spores 14.44 99.2% Influenza virus Virus 2.92 >99,9 % SARS-CoV-2 Virus 2.73 >99,9 % ‡ The stand-alone UV222 Booth is programmed for a 30 sec. cycle. This can be adapted to different requirements.
-
Material transmission
Far UV-C transmission was found to be less than 0.05% for all protective equipment used at a pilot installation site. Therefore, the dose reaching the skin and eyes of the gowned operator would be 0.0058 mJ/cm² per 30 sec. cycle, well below the allowed daily exposure (Threshold Limit Value of 22 mJ/cm²), even after multiple uses.
Testing should be done with the specific protective equipment used at the installation site to ensure safety of gowned workers using the UV222 Booth.
-
Dimensions
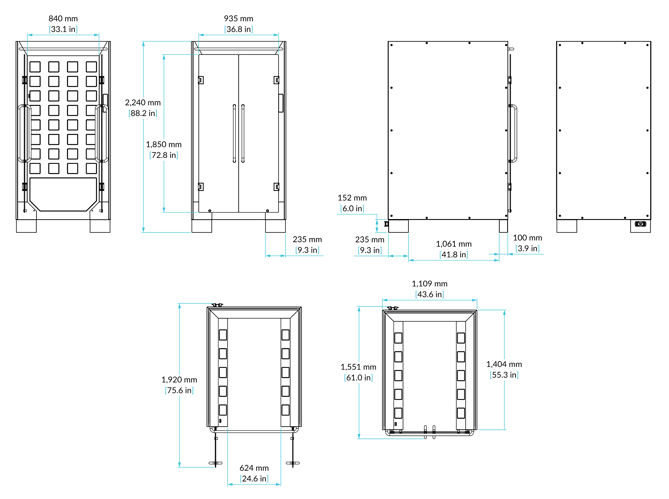
Dimensions
Dimensions are based on a standard booth, but it can be customised to your needs.
-
Installation & maintenance

Installation & maintenance
The UV222 Booth is designed for easy installation and maintenance. The different parts and components are packed individually for safe transport, and assembled in the final facility. The surface is accessible for cleaning and can be ordered in one of two types of medical-grade stainless steel (304L or 316L), renowned for its strength, durability, and corrosion resistance.
Contact us for more information.
-
Downloads
Do you want the Product Datasheet or the Technical & Installation manual?

Introducing Mercury-Free Far-UVC Technology with KrCl Lamps
UV Medico is proud to introduce an advanced disinfection technology: mercury-free, 222 nm Krypton-Chloride (KrCl) excimer lamps. These innovative lamps offer a safe, sustainable alternative to traditional mercury-based systems, aligning with modern environmental and health standards. Ideal for cleanroom environments, KrCl lamps assure high-level disinfection efficacy without compromising human or ecological safety.
Get in touch with UV Medico
Should you be keen to explore the capabilities of the UV222 Booth, just fill in your email. Our team will connect with you promptly, offering insights and support tailored to your needs.
Comply with Annex 1 using the groundbreaking UV222 Booth
The new Annex 1, taking effect on August 25, 2023, mandates that manufacturers of sterile products must implement measures to minimize the risk of microbial contamination.
The UV222 Booth aids compliance with Annex 1 by effectively inactivating microorganisms on gowned personnel before they enter high-grade cleanroom areas.


A Deep Dive into the UV222 Booth: A Leap in Decontamination Technology
UV Medico's UV222 Booth revolutionizes cleanroom sanitization with its Far-UVC light technology. This innovative booth provides a quick, 21-second disinfection process for personnel, seamlessly integrating into existing controlled environments without compromising safety or efficiency.
Latest Cleanroom knowledge from UV Medico
3 min read
How a New Light Emerged: The Rise of Far-UVC in Disinfection Science
Apr 22, 2025 by Emilie Hage Mogensen

 UV222™
UV222™ UV222 Linear
UV222 Linear UV222 Downlight
UV222 Downlight Vertex 222
Vertex 222.png) UV222 Pendant
UV222 Pendant.png) UV222 Booth
UV222 Booth.png) UV222 Step-On
UV222 Step-On.png) UV222 Cleanroom Downlight
UV222 Cleanroom Downlight UV222 Material Airlock
UV222 Material Airlock UV222 Ambulance
UV222 Ambulance UV222 Industrial
UV222 Industrial